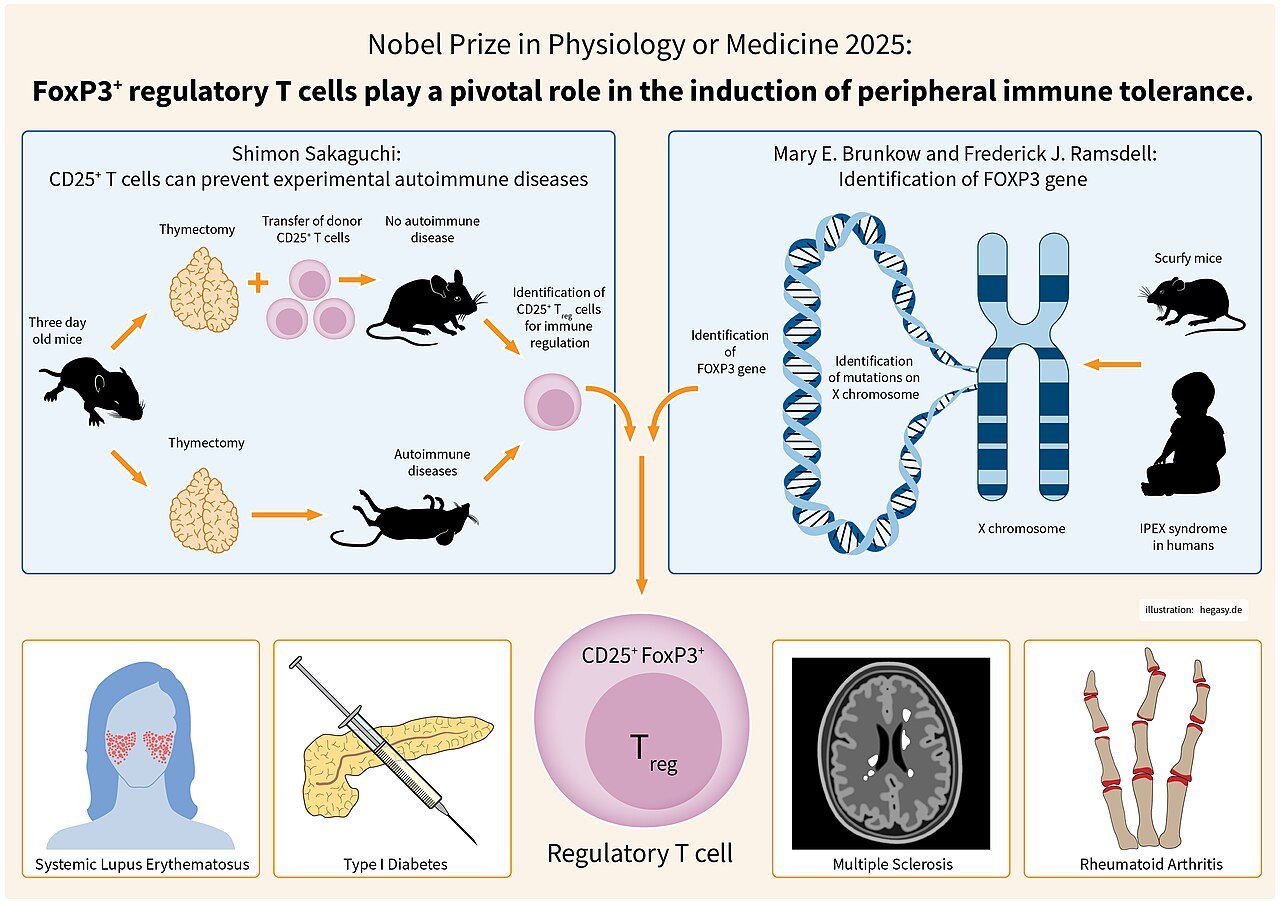
Mary Elizabeth Brunkow (born 1961) is an American molecular biologist and program supervisor whose pioneering analysis uncovered the genetic foundation of immune regulation. Greatest recognized for her co-discovery of the FOXP3 gene, a central regulator of regulatory T (Treg) cell improvement, Brunkow’s work laid the molecular basis for understanding immune tolerance — the physique’s skill to differentiate self from non-self. In recognition of this transformative contribution, she shared the 2025 Nobel Prize in Physiology or Drugs with Fred Ramsdell and Shimon Sakaguchi “for his or her discoveries regarding peripheral immune tolerance.”
Early Life and Training
Born and raised within the Pacific Northwest, Brunkow developed a deep curiosity about biology from an early age. She attended the College of Washington, the place she earned her Bachelor of Science in Cell and Molecular Biology (1979–1983). The rigorous educational and analysis atmosphere at UW launched her to molecular genetics and laboratory science, sparking her lifelong curiosity in gene regulation and immunology.
Brunkow continued her educational journey at Princeton College, incomes her Ph.D. in Molecular Biology (1984–1990). At Princeton, she was educated in cutting-edge strategies of molecular genetics, engaged on the regulation of gene expression in mammalian programs. This expertise outfitted her with a mixture of scientific depth and technical precision that might grow to be the hallmark of her later discoveries.
Early Profession and the FOXP3 Discovery
After finishing her doctorate, Brunkow joined Celltech R&D, Inc. in 1994 as a Senior Scientist and later Director of Genomics, the place she spent a decade (1994–2004) conducting revolutionary analysis in molecular immunology and purposeful genomics. At Celltech, she was concerned in research that examined immune dysfunction in murine fashions — notably a pressure of mice often known as scurfy mutants, which developed a extreme autoimmune syndrome.
Brunkow and her colleagues undertook positional cloning of the gene chargeable for the scurfy phenotype and found FOXP3, a member of the forkhead/winged-helix household of transcription elements. Her 2001 publication demonstrated that mutations in FOXP3 brought on the lack of purposeful regulatory T cells, resulting in uncontrolled immune activation. Quickly after, parallel findings in people revealed that mutations in FOXP3 end in IPEX syndrome (Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked) — a deadly autoimmune dysfunction.
This pivotal discovery supplied molecular proof for the existence of a genetically outlined subset of T cells chargeable for immune tolerance, remodeling immunology. The identification of FOXP3 and Tregs grew to become a cornerstone of contemporary immunotherapy, influencing remedies for autoimmune ailments, organ transplantation, and most cancers.
Translating Science into Software
Following her tenure at Celltech, Brunkow transitioned into biotech and translational analysis roles that bridged primary science with therapeutic improvement. In 2004, she served briefly as a Senior Scientist (contract) at Blue Heron Biotechnology, making use of her molecular experience to DNA synthesis and genomic evaluation.
In 2006, she joined the Institute for Programs Biology (ISB) as a Science Author, reflecting her skill to speak advanced scientific concepts throughout disciplines. She quickly returned to analysis administration, taking up more and more senior roles within the biotechnology sector.
From 2008 to 2010, she served as Affiliate Director of Program Administration at Trubion Prescribed drugs, a Seattle-based biopharmaceutical firm creating focused biologics for autoimmune and inflammatory ailments. At Trubion, she managed interdisciplinary analysis packages centered on antibody engineering and immune modulation, persevering with her career-long engagement with translational immunology.
Management on the Institute for Programs Biology
Since June 2009, Dr. Brunkow has held the place of Program Supervisor for Genetics on the Institute for Programs Biology (ISB) in Seattle, Washington. Over greater than 16 years, she has performed a central function in coordinating analysis packages that combine genomics, programs biology, and computational modeling. ISB’s mission — to grasp biology as an built-in and dynamic system — aligns carefully with Brunkow’s imaginative and prescient of connecting molecular mechanisms to physiological outcomes.
Her management at ISB has prolonged into challenge design, information integration, and scientific communication. She has guided cross-functional groups of researchers, bioinformaticians, and clinicians to sort out advanced organic questions, from immune response profiling to precision medication approaches.
Key Challenge: Immune Repertoire Sequencing for Sepsis Detection
Amongst Brunkow’s notable ISB initiatives is her management within the “Pre-symptomatic Analysis of Sepsis through Excessive-Throughput Immune Repertoire Sequencing (Rep-Seq)” challenge (initiated in 2013). The challenge explores how immune repertoire range — the number of T and B cell receptors — adjustments in response to an infection or stress.
Sepsis stays a number one reason for mortality in hospitalized sufferers, largely because of delayed prognosis. Brunkow’s challenge hypothesizes that early adjustments in immune repertoire range can function a delicate biomarker for sepsis, even earlier than medical signs seem. Utilizing high-throughput sequencing of CDR3 areas from T-cell and B-cell receptors, her staff measures shifts in immune range throughout serial affected person samples. The strategy represents an revolutionary systems-level utility of immunogenomics — merging her foundational experience in immune tolerance with real-world diagnostic innovation.
This analysis displays Brunkow’s ongoing curiosity in host-pathogen interactions, immune system dynamics, and biomarker discovery — all central to trendy precision medication.
Skilled Abilities and Influence
Dr. Brunkow’s skilled profile combines deep scientific information with robust organizational and collaborative expertise. Her experience spans:
-
Genetics and Genomics
-
Immunology and Molecular Biology
-
Biotechnology Improvement
-
Program and Challenge Administration
-
Scientific Communication and Writing
She has been endorsed by quite a few colleagues for her technical and management strengths in Genetics and Biotechnology, with a number of endorsements from friends on the Institute for Programs Biology. Her scientific contributions, coupled along with her skill to handle massive interdisciplinary groups, have made her a revered determine in each educational and biotech communities.
Recognition and the Nobel Prize
In 2025, the Nobel Meeting acknowledged Mary E. Brunkow, Fred Ramsdell, and Shimon Sakaguchi with the Nobel Prize in Physiology or Drugs for his or her work elucidating the mechanisms of peripheral immune tolerance. The Nobel Committee cited Brunkow’s discovery of FOXP3 as a “genetic key to understanding the immune system’s self-regulatory capability.” Her findings helped clarify how the immune system prevents self-destruction and supplied a framework for creating therapies that harness or modulate Tregs.
The popularity introduced renewed consideration to her profession trajectory — from elementary molecular biology to translational immunology and systems-level biomedical analysis. Universities and scientific establishments praised her collaborative spirit and her emphasis on integrating numerous disciplines to unravel organic issues.
Private Life and Legacy
Primarily based in Seattle, Washington, Dr. Brunkow stays lively in mentoring and supporting younger scientists, notably in genetics and immunology. Colleagues describe her as modest, exact, and deeply dedicated to scientific rigor.
Her legacy is outlined not solely by the FOXP3 discovery, which redefined immunology, but in addition by her sustained efforts to use systems-level considering to medication. From revealing the genetic foundations of immune tolerance to creating genomic diagnostics for sepsis, Dr. Brunkow’s work exemplifies the combination of discovery science and translational affect.

